FTC warns pharma companies it means business with its Orange Book listing policy
- May 15, 2024
- Snippets
Last September, the Federal Trade Commission (FTC) promulgated a “policy statement” entitled “Statement Concerning Brand Drug Manufacturers’ Improper Listing of Patents in the Orange Book,” regarding the FTC’s allegations that some patents in the FDA’s Orange Book were improperly listed because they were neither new drugs nor methods of using them, but rather were directed to medical devices. Recently the FTC sent letters almost identical in substantive import to ten major drug companies (Amphaster Pharma, Covis Pharma, Novartis, Norton (Waterford) Ltd., GlaxoSmithKline, Glaxo Group Ltd. Boehringer Ingelheim, AstraZeneca AB, Novo Nordisk and Teva Pharma) regarding particular allegations in this regard. The letters recited each patent that the FTC alleged were improperly listed and the New Drug Applications (NDAs)/drugs corresponding to the listings. The FTC’s justifications for the letters in each case were that “patents improperly listed in the Orange Book may delay lower-cost generic drug competition” [by inducing the 30-month stay of approval under 21 U.S.C. §§ 355(c)(3)(C), (j)(5)(B)(iii) and] “in addition to delays resulting from such a stay of approval, the costs associated with litigating improperly listed patents may disincentivize investments in developing generic drugs, which risks delaying or thwarting competitive entry.”
The letters further warn that the FTC has chosen to use the statutory pathway under 21 C.F.R. 314.53(f)(1)(i)(A) for disputing “the accuracy or relevance of patent information submitted” to the FDA for Orange Book listing but that the Commission “retain[s] the right to take any further action the public interest may require, which may include investigating this conduct as an unfair method of competition under Section 5 of the FTC Act, 15 U.S.C. § 45.” These stratagems enable imposition of civil (monetary) penalties, but an earlier warning in the Policy Statement itself that “if the FTC encounters false certifications filed under 21 C.F.R. § 314.53(c)(2)(ii)(R) that may constitute a potential criminal violation for the submission of false statements, the Commission may refer such cases to the U.S. Department of Justice for further investigation” has not been abjured.
This latest action raises the question: why these companies, and what behavior prompted the FTC to act now? Each letter contains a table of the patents that the FTC calls into question, summarized as follows herein:
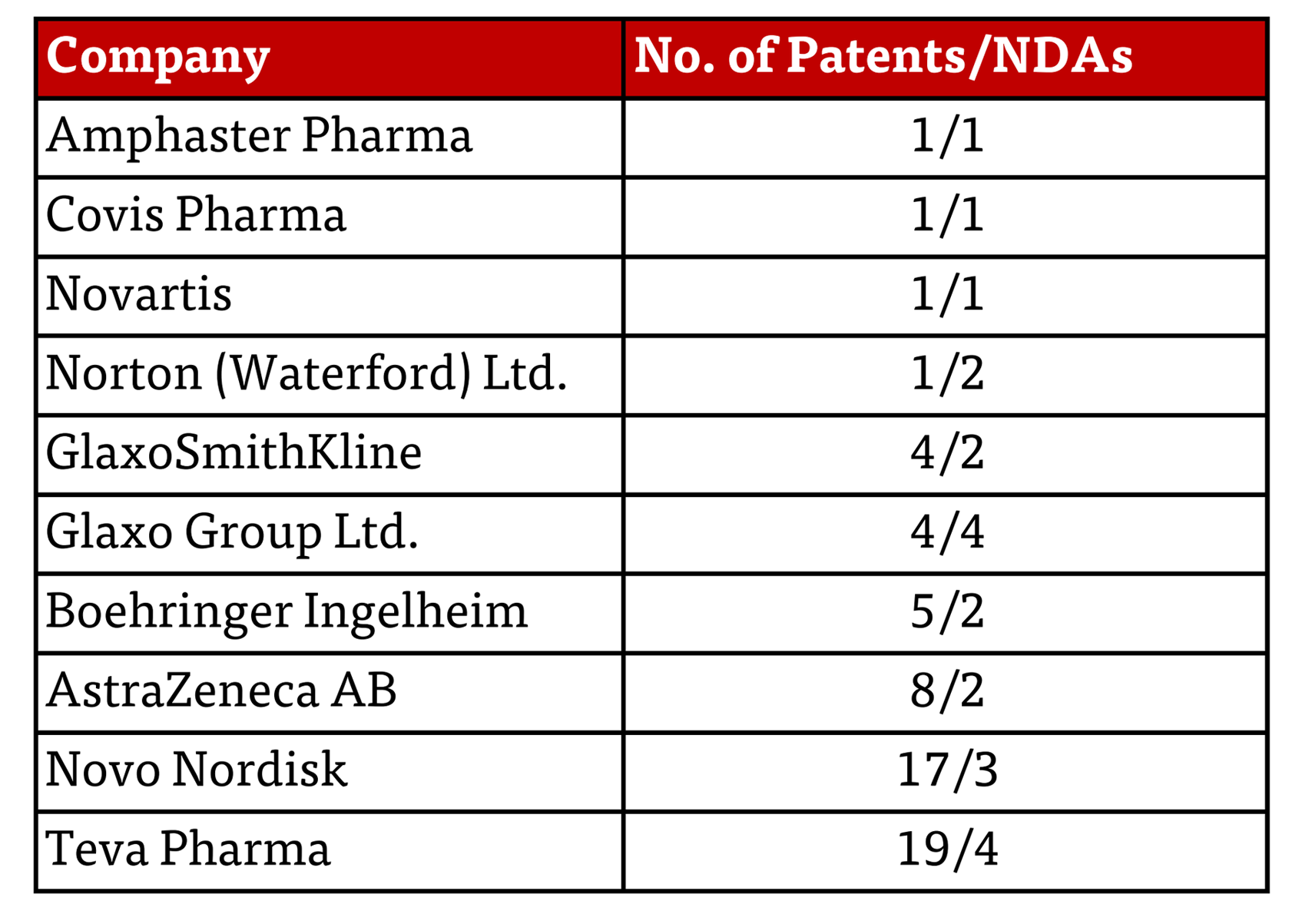
A closer look shows that what these patents protect are, by and large, medical devices that provide either greater accuracy in administered dose or increased patient convenience and accompanying greater patient compliance; see, for example, U.S. Patent No. 7,654,986 (expiration expected July 12, 2024)(assigned to Novo Nordisk); U.S. Patent No. 8,161,968 (expiration expected February 5, 2028)(GSK); and U.S. Patent No. 8,182,838 (expiration expected October 20, 2028)(Novartis).
A close look at the frequency with which these patents have been asserted in an abbreviated new drug application (ANDA) litigation shows that most of them have not been so asserted. Of the 61 patents identified in the FTC’s letters (presumably indicating some level of differentiation to select somewhat- to particularly egregious-examples of bad behavior) 34 have not been asserted and eight others have been asserted in only five ANDA cases. The anomaly is 19 patents asserted in 209 cases, with the majority of these being directed to Ozembic, Saxenda, and Victoza. Whether this exemplifies over-exuberance, bad acting, or justifiable protection of devices providing patient-specific advantages remains to be determined.
The response from each of these seven pharmaceutical companies to the FTC’s mandate will vary, no doubt (and asserting these patents in ANDA litigation is likely imprudent when routine avenues of recourse may suffice). But these alternatives may involve a higher degree of disruption of markets and patient-availability of the medicines under NDA and reduce investment into new and better ways of administering drugs known to be more difficult in this regard than conventional drugs, or that would provide real-world benefit for patients. Because the Policy Statement and the FTC’s actions in enforcing it are just the early stages of their regulatory effort, it is impossible to predict the outcome. But if earlier FTC ideology-based efforts (such as their crusade against reverse-settlement agreements a decade ago) are a guide, it is likely to be a protracted struggle.

